| 1 |
MD 1 – Application for grant of Certificate of Registration of a Notified Body |
MD 2 – Certificate of Registration for a Notified Body under the Medical Devices Rules, 2017 |
NA |
Rs.25000/- |
 |
|
| 2 |
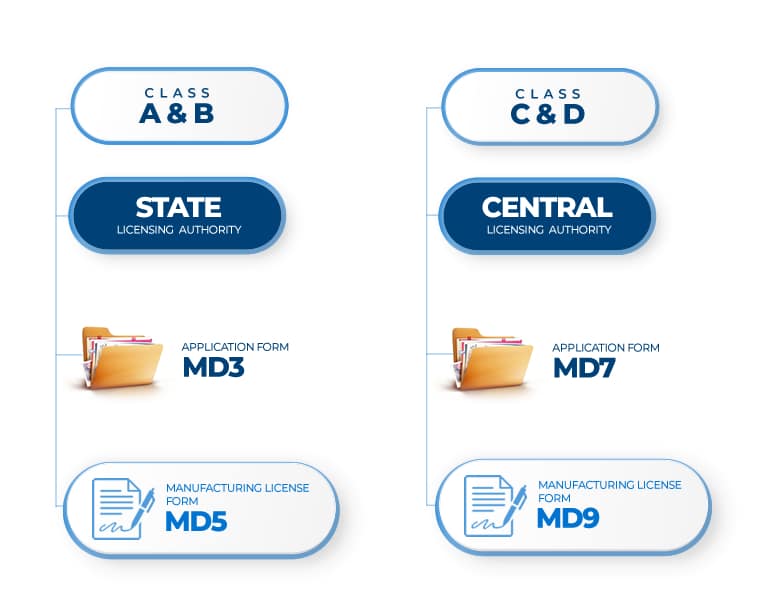
MD 3 – Application for Grant of Licence to Manufacture for Sale and Distribution of Class A or Class B medical device |
MD 5 – Licence to Manufacture for Sale or for Distribution of Class A or Class B Medical Device. |
A & B |
Rs.5000 for plant registration
Rs.500 for product (per product) |
|
 |
| 3 |
MD 4 – Application for Grant of Loan Licence to Manufacture for Sale or for Distribution of Class A or Class B medical device |
MD 6 – Loan Licence to Manufacture for Sale or for Distribution of Class A or Class B medical device |
A & B |
Rs.5000 for plant registration
Rs.500 for product (per product) |
|
 |
| 4 |
MD 7 – Application for Grant of Licence to Manufacture for Sale or for Distribution of Class C or Class D |
MD 9 – Licence to Manufacture for Sale or for Distribution of Class C or Class D |
C & D |
Rs.50000 for plant registration
Rs.1000 for product (per product) |
 |
|
| 5 |
MD 8 – Application for Grant of Loan Licence to Manufacture for Sale or for Distribution of Class C or Class D |
MD 10 – Loan Licence to Manufacture for Sale or for Distribution of Class C or Class D medical device |
C & D |
Rs.50000 for plant registration
Rs.1000 for product (per product) |
|
 |
| 6 |
MD 11 – Form in which the Audit or Inspection Book shall be maintained |
|
NA |
$6000 |
 |
|
| 7 |
MD 12 – Application for licence to manufacture medical device for purpose of clinical investigations, test, evaluation, examination, demonstration or training |
MD 13 – Licence to Manufacture Medical Devices for the Purposes of Clinical Investigations or Test or Evaluation or Demonstration or Training |
A,B,C,D |
Rs.500/- |
 |
 |
| 8 |
MD 14 – Application for issue of import licence to import medical device In Class A (Other than in vitro) |
MD 15 – Licence to Import Medical Device In Class A (other than in vitro) |
A |
$1000 for plant
$50 for product (per product) |
 |
|
| 9 |
MD 14 – Application for issue of import licence to import medical device In Class B (Other than in vitro) |
MD 15 – Licence to Import Medical Device In Class B (other than in vitro) |
B |
$2000 for plant
$1000 for product (per product) |
 |
|
| 10 |
MD 14 – Application for issue of import licence to import medical device In Class C or D (Other than in vitro) |
MD 15 – Licence to Import Medical Device In Class C or D (other than in vitro) |
C & D |
$3000 for plant
$1500 for product (per product) |
 |
|
| 11 |
MD 14 – Application for issue of import licence to import medical device In Class A or B (In vitro) |
MD 15 – Licence to Import Medical Device In Class A or B (In vitro) |
A & B |
$1000 for plant
$10 for product (per product) |
 |
|
| 12 |
MD 14 – Application for issue of import licence to import medical device In Class C or D (In vitro) |
MD 15- Licence to Import Medical Device In Class C or D (In vitro) |
C & D |
$3000 for plant
$500 for product (per product) |
 |
|
| 13 |
MD 16 – Application for Licence to Import Medical Devices for the Purposes of Clinical Investigations or Test or Evaluation or Demonstration or Training |
MD 17 – Licence to Import Medical Devices for the Purposes of Clinical Investigations or Test or Evaluation or Demonstration or Training |
A,B,C,D |
$100 |
 |
|
| 14 |
MD 18 – Application for licence to import investigational medical devices for the purposes by a government hospital or statutory medical institution for the treatment of patients |
MD 19 – Licence to import investigational medical device by a government hospital or statutory medical institution for the treatment of patients |
A,B,C,D |
Rs.500/- |
 |
|
| 15 |
MD 20 – Application for permission to import small quantity of medical devices for personal use |
MD 21 – Permission to import of small quantity of medical devices for personal use |
A,B,C,D |
No fee |
 |
|
| 16 |
MD 22 – Application for Grant of permission to conduct clinical investigation of an investigational medical device |
Form 23 – Permission to conduct Clinical Investigation |
A,B,C,D |
Rs.100000/- |
 |
|
| 17 |
MD 24 – Application for grant of permission to conduct clinical performance evaluation of new in vitro diagnostic medical device |
MD 25 – Permission to conduct clinical performance evaluation of new in vitro diagnostic medical device |
A,B,C,D |
Rs.25000/- |
 |
|
| 18 |
MD 26 – Application for grant of permission to import / manufacture for sale or for distribution of medical device which does not have predicate medical device |
MD 27 – Permission to import or manufacture for sale or for distribution of medical device which does not have predicate medical device |
A,B,C,D |
Rs.50000/- |
 |
|
| 19 |
MD 28 – Application for grant of permission to Import or Manufacture for sale or for distribution of new in vitro diagnostic medical device |
MD 29 – Permission to Import or Manufacture New In Vitro Diagnostic Medical Device |
A,B,C,D |
Rs.25000/- |
 |
|
| 20 |
MD 39 – Application for grant of registration to Medical Device Testing Laboratory for carry out Test or Evaluation of a medical device on behalf of manufacturer |
MD 40 – Certificate of registration to Medical Device Testing Laboratory for carry out Test or Evaluation of a medical device on behalf of manufacturer |
NA |
Rs.20000/- |
 |
|